Solar Energy International
Biodiesel Workshop
Diesel Vocabulary
• Aftercooling / Intercooling
• Turbocharging
• Cetane Number
• Cloud Point (CP)
• Flash Point
• Cold Filter Plugging Point (CFPP)
• Pour Point
• Compression Ignition (CI)
• Direct Injection (DI)
• In-Direct Injection (IDI)
• In-Line Injection Pump
• Nitrogen Oxides (NOx)
• Pump-Line-Nozzle Fuel System
• Rotary Injection Pump
• Unit Injector
• Common Rail Injection
What is a Diesel Engine?
• Rudolf Diesel developed the idea for the diesel engine and obtained the German patent for it in 1892.
• His goal was to create an engine with high efficiency.
• Gasoline engines had been invented in 1876 and, especially at that time, were not very
efficient
• Both the gasoline and diesel engine utilize the process of
internal combustion for power
What is Internal Combustion?
Four stroke cycle
• Intake stroke:
intake valve opens while the piston moves down from its highest position in the cylinder to its lowest position, drawing air into the cylinder in the process.
• Compression stroke:
intake valve closes and the piston moves back up the cylinder. This compresses the air & therefore heats it to a high temperature, typically in excess of 1000°F (540°C). Near the end of the compression stroke, fuel is injected into the cylinder. After a short delay, the fuel ignites spontaneously, a process called auto ignition.
• Combustion stroke:
The hot gases produced by the combustion of the fuel further increase the pressure in the cylinder, forcing the piston down
• Exhaust stroke:
exhaust valve opens when the piston is again near its lowest position, so that as the piston once more moves to its highest position, most of the burned gases are forced out of the cylinder.
Biodiesel Workshop
Diesel Vocabulary
• Aftercooling / Intercooling
• Turbocharging
• Cetane Number
• Cloud Point (CP)
• Flash Point
• Cold Filter Plugging Point (CFPP)
• Pour Point
• Compression Ignition (CI)
• Direct Injection (DI)
• In-Direct Injection (IDI)
• In-Line Injection Pump
• Nitrogen Oxides (NOx)
• Pump-Line-Nozzle Fuel System
• Rotary Injection Pump
• Unit Injector
• Common Rail Injection
What is a Diesel Engine?
• Rudolf Diesel developed the idea for the diesel engine and obtained the German patent for it in 1892.
• His goal was to create an engine with high efficiency.
• Gasoline engines had been invented in 1876 and, especially at that time, were not very
efficient
• Both the gasoline and diesel engine utilize the process of
internal combustion for power
What is Internal Combustion?
Four stroke cycle
• Intake stroke:
intake valve opens while the piston moves down from its highest position in the cylinder to its lowest position, drawing air into the cylinder in the process.
• Compression stroke:
intake valve closes and the piston moves back up the cylinder. This compresses the air & therefore heats it to a high temperature, typically in excess of 1000°F (540°C). Near the end of the compression stroke, fuel is injected into the cylinder. After a short delay, the fuel ignites spontaneously, a process called auto ignition.
• Combustion stroke:
The hot gases produced by the combustion of the fuel further increase the pressure in the cylinder, forcing the piston down
• Exhaust stroke:
exhaust valve opens when the piston is again near its lowest position, so that as the piston once more moves to its highest position, most of the burned gases are forced out of the cylinder.
Four stroke Cycle

Gasoline versus Diesel
• Spark ignition:
Gasoline engines use spark plugs to ignite fuel/ air mixture
• Compression ignition:
Diesel engines uses the heat of compressed air to ignite the fuel (intakes air, compresses it, then injects fuel)
• Fuel injection:
-Gasoline uses port fuel injection or carburetion;
-Diesel uses direct fuel injection or pre combustion chambers (indirect injection)
• Glow plugs:
-electrically heated wire that helps heat pre combustion chambers fuel when the engine is cold
- when a diesel engine is cold, compression may not raise air to temperature needed for fuel ignition

Gasoline versus Diesel
• Spark ignition:
Gasoline engines use spark plugs to ignite fuel/ air mixture
• Compression ignition:
Diesel engines uses the heat of compressed air to ignite the fuel (intakes air, compresses it, then injects fuel)
• Fuel injection:
-Gasoline uses port fuel injection or carburetion;
-Diesel uses direct fuel injection or pre combustion chambers (indirect injection)
• Glow plugs:
-electrically heated wire that helps heat pre combustion chambers fuel when the engine is cold
- when a diesel engine is cold, compression may not raise air to temperature needed for fuel ignition
Compression Ratio
• Compression ratio:
This is defined as the ratio of the volume of the cylinder at the beginning of the compression stroke (when the piston is at BDC) to the volume of the cylinder at the end of the compression stroke (when the piston is at TDC).
• The higher the compression ratio, the higher the air temperature in the cylinder at the end of the compression stroke.
• Higher compression ratios, to a point, lead to higher thermal efficiencies and better fuel economies.
• Diesel engines need high compression ratios to generate the high temperatures required for fuel auto ignition.
• In contrast, gasoline engines use lower compression ratios in order to avoid fuel auto ignition, which manifests itself as engine knock or pinging sound.
• Common spark ignition compression ratio: 8:1 to 12:1
• Common compression ignition ration: 14:1 to 25:1
Direct Injection vs. Indirect
Injection
Injection
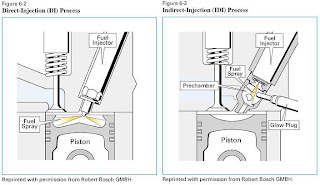
Direct Injection

• Direct-Injection (DI) or Open Chamber Engine: In this design, the fuel is injected directly into the cylinder chamber.
Direct injection engines have two design philosophies:
-High-swirl design, which have a deep bowl in the piston, a low number of holes in the injector and moderate injection pressures.
-Low-swirl or quiescent engines are characterized by having a shallow bowl in the piston, a large number of holes in the injector and higher injection pressures.
• Smaller engines tend to be of the high-swirl type, while bigger engines tend to be of the quiescent type
• All newer diesel engines use direct fuel injection
• Much higher fuel pressure then indirect fuel injection (example TDI )
• Injection/Injector Timing is critical
• Equipped with in-line pumps, distributor pumps, rail injection systems, or pump injector units
In this design, the fuel is injected into a small pre-chamber attached to the main cylinder chamber.
The combination of rapidly swirling air in the prechamber and the jet-like expansion of combustion gases from the prechamber into the cylinder enhances the mixing and combustion
of the fuel and air.
Starting is aided by a high compression ratio (24-27) and a glow plug mounted in the pre-chamber.
This design has the advantage of less noise and faster combustion, but typically suffers from poorer fuel economy.
Direct injection engines have two design philosophies:
-High-swirl design, which have a deep bowl in the piston, a low number of holes in the injector and moderate injection pressures.
-Low-swirl or quiescent engines are characterized by having a shallow bowl in the piston, a large number of holes in the injector and higher injection pressures.
• Smaller engines tend to be of the high-swirl type, while bigger engines tend to be of the quiescent type
• All newer diesel engines use direct fuel injection
• Much higher fuel pressure then indirect fuel injection (example TDI )
• Injection/Injector Timing is critical
• Equipped with in-line pumps, distributor pumps, rail injection systems, or pump injector units
In this design, the fuel is injected into a small pre-chamber attached to the main cylinder chamber.
The combination of rapidly swirling air in the prechamber and the jet-like expansion of combustion gases from the prechamber into the cylinder enhances the mixing and combustion
of the fuel and air.
Starting is aided by a high compression ratio (24-27) and a glow plug mounted in the pre-chamber.
This design has the advantage of less noise and faster combustion, but typically suffers from poorer fuel economy.